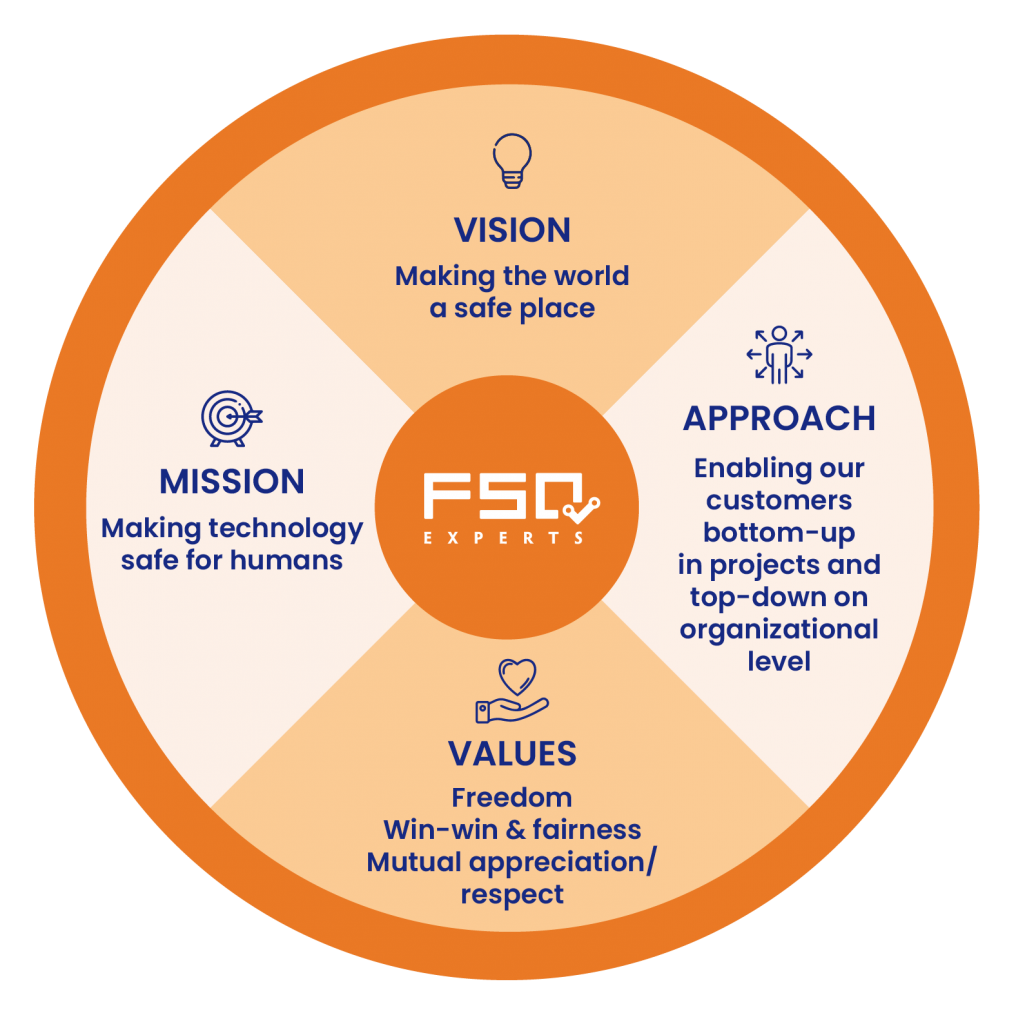
OUR COMPETENCES
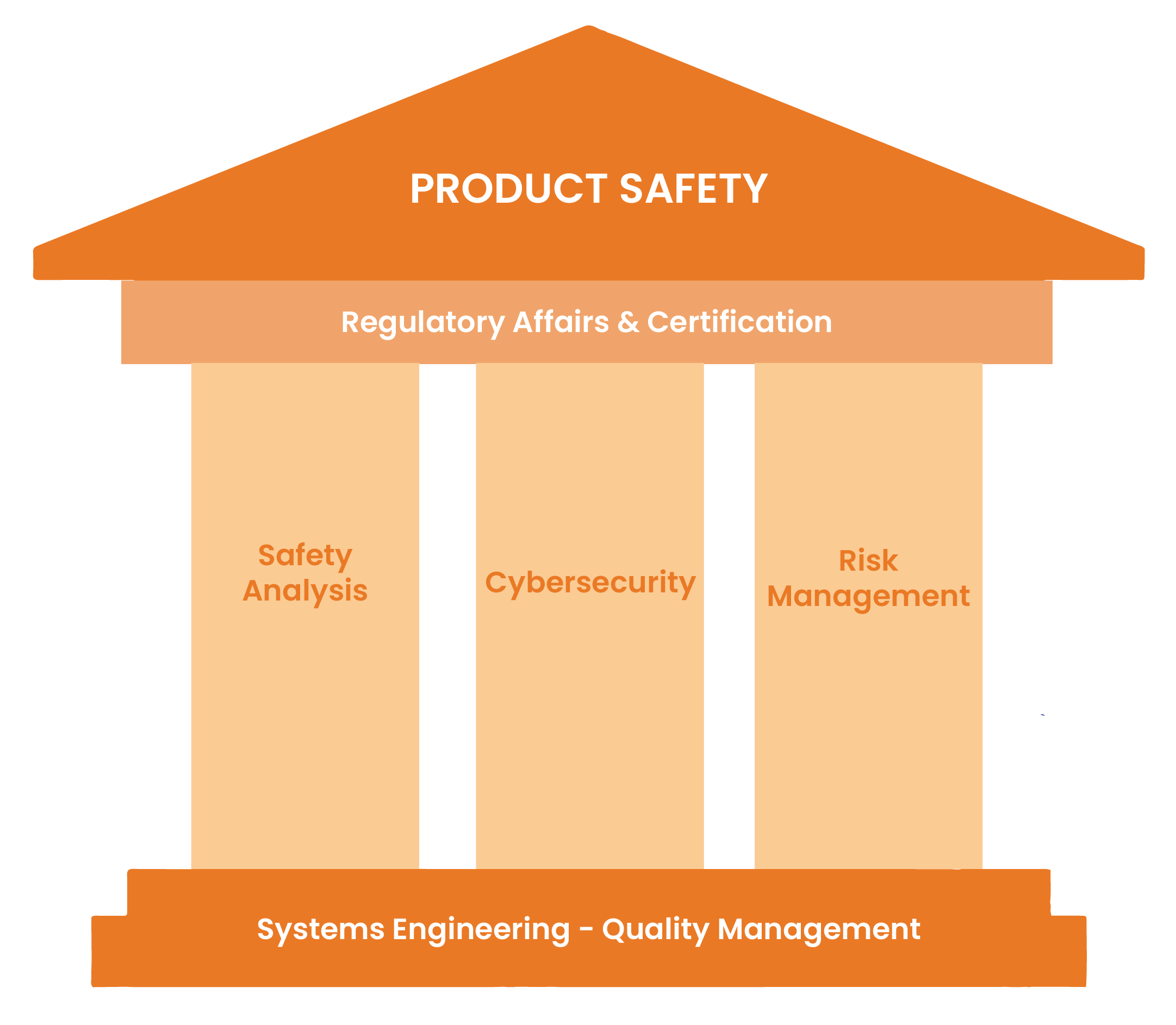
Systems Engineering for safety relevant products
We have worked with original equipment manufacturers (OEMs), suppliers and
start-ups implementing development processes for safety relevant products at
company level and at project level. With our expertise on product safety, V-Model and
Agile methodologies these organizations achieved a company wide safety culture and
the implementation of development processes compliant with industry standards such
as ISO 26262, ARP4754 and IEC 60601-1
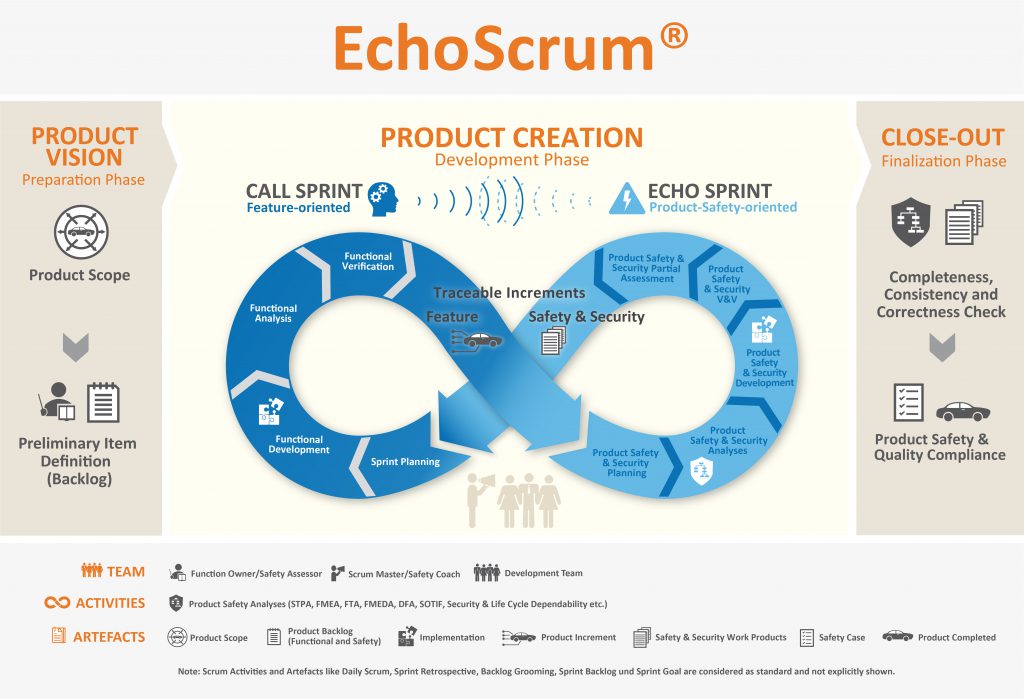
Check out EchoScrum our solution based on ISO26262 + Agile Methodologies
Safety Analysis & Risk Management
We have partnered with several customers to help them implement and perform safety analysis methods. Our expertise, workshop based approaches and customized analysis tools have been proven to be very succesful to accelerate the delivery of complex analyses.
In addition, we manage the risks derived from these analyes thourought the development life cycle and define strategies to monitor the products during their operation life cycle.
Quality Management
Our team of experts work in our customer’s premises as interim Quality Managers providing active hands-on coaching. With this dynamic approach these customers have successfully built and improved their QM system and passed audits without findings.
Certification / Regulatory affairs
We support international companies to reduce the “time to market” of their safety-related products defining regulatory strategies and certification roadmaps. The key to this success is the fruitful collaboration between our customers and FSQ’s legal experts on industry-specific laws, regulations and international technical standards.
We support the deployment of products in: North America, european region and china.
OUR SERVICES
 AUTOMOTIVE
AUTOMOTIVE
Safe Product development
- Implementation of development process according to ISO 26262 and ISO/PAS 21448
- Implementation of Agile safety oriented solutions such as:
EchoScrum - Coaching, workshops and direct support defining strategies to
establish a solid safety culture.
Field Operation, Monitoring & configuration updates
- Planning Field Operation & Monitoring activities
- Defining strategies to assess the risk of vehicle functional updates. (E.g: Software updates)
- Defining strategies to collect field data
Safety Analysis & Risk management
- Creation of Work products according to ISO26262: Item definition,
plans, Safety case, etc. - Creation of Safety analyses HARA, HiRe, FSP, FSC, TSC, FMEA,
FMEDA, DFA, STPA, TARA - Coaching on how to perform safety analyses
- Customized tools to automatize safety analyses
- Safety management according to ISO26262
Quality management
- Implementation of Quality Management process
- Performance of gap analyses for compliance with:
- ISO9001:2015
- IATF 16949:2016
- Automotive SPICE® v3.1
 MEDICAL DEVICES
MEDICAL DEVICES
Quality Management
- Coaching on implementation of a QMS according to ISO 13485 for start-ups
- Interim Quality Management for start-ups and middle-size companies
- Support on preparation of ISO 13485 and/or MDR Audits
Development Lifecycle
- Requirements Engineering
- Coordination of Verification Activities (e.g.Packaging Validation, EMC Testing,)
- Moderation of risk management workshops
- Interim Risk Management (ISO 14971) for start-ups and middle-size companies
- Reliability Engineering (e.g. MTTF analysis,Moderation of FMEA,…)
Certification and Regulatory Affairs
- Assessment of medical device classes on target markets
- Planning regulatory strategy for CE Markt, USA and
China - Support for technical documentation for submissions to notified bodies (STED File, PTR)
- Review of claims matrices, IFU or marketing brochures
- Support for renewal of product registrations
Post Market Surveillance (PMS)
- Planning Post-Market Surveillance (PMS) Activities
- Defining strategies for field data collection (e.g.supported by IT cloud solutions)
- Review of PMS Reports
 AEROSPACE
AEROSPACE
Product development
- Implementation of dev. processes:
- Aircraft & System level acc. to ARP 4754A
- SW and HW levels acc. to DO178C, DO 254
- Support & coaching on engineering tasks:
- Preparation of plans.
- Requirements Engineering.
- Development Assurance management.
- Configuration Management.
Product safety
- Creation of Safety assessments acc to ARP 4761 including:
- Safety Program Plans
- PSSA and SSA
- Safety analyses:
- FHAs, FTAs, PRAs, CMAs & ZSAs
- Risk assessments for specific operation RPAS: SORA
Certification and Airworth. regulations
- Support for compliance with
- Aircraft regulations: CS-23, CS-25.
- RPAS regulations: EASA Rules for UAS.
- VTOL special conditions: SC-VTOL-01.
- EASA Part 21
Quality management & Development Assurance
- Gap analyses and preparation for audits:
- System Level: ARP 4754A.
- HW and SW Level: DO178C & Do254.
- Implementation of QM Systems (ISO 9001, EN 9100).
SUCCESS STORIES
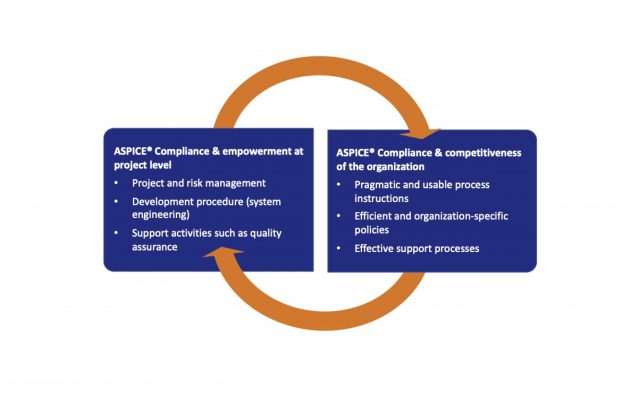
ASPICE
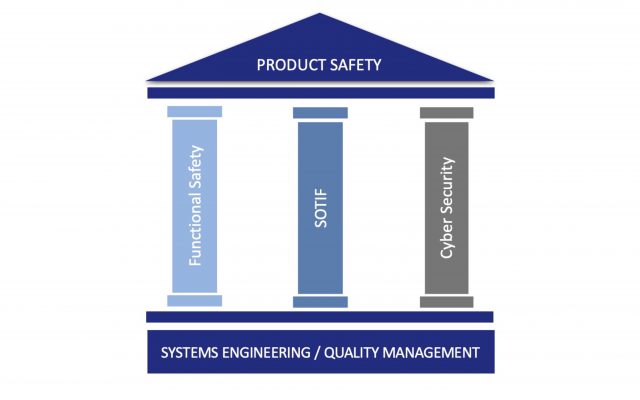
Functional Safety
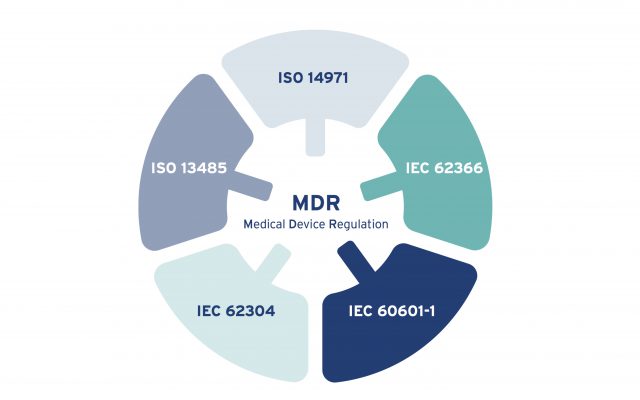
Medical Device
SOLUTIONS
MEET US
CONTACT & LOCATION
FSQ Experts (Germany)
FSQ Experts North America Inc. (Canada)